Total Organic Carbon (TOC) and its Measurement
15 Apr 2021
Dr Paul Whitehead
After a BA in Chemistry at Oxford University, Paul focused his career on industrial applications of chemistry. He was awarded a PhD at Imperial College, London for developing a microwave-induced-plasma detector for gas chromatography. He spent the first half of his career managing the analytical support team at the Johnson Matthey Research/Technology Centre,specialising in the determination of precious metals and characterising applications such as car-exhaust catalysts and fuel cells. Subsequently, as Laboratory Manager in R&D for ELGA LabWater, he has been involved in introducing and developing the latest water purification technologies. He now acts as a consultant for ELGA.
What is Total Organic Carbon (TOC)?
Total Organic Carbon (TOC) is a measure of the total amount of carbon in organic compounds in pure water and aqueous systems. TOC is a valued, analytical technique that is applied by organizations and labs to determine how suitable a solution is for their processes. Unless it’s ultrapure, water will naturally contain some organic compounds, understanding how much is key.
TOC has become an important parameter used to monitor overall levels of organic compounds present. This has happened despite the lack of any direct quantitative correlation between total organic carbon and the total concentration of organic compounds present and reflects the importance of having an easy-to-measure, general indicator of the approximate level of organic contamination.
It also reflects the appeal of a parameter which has a name which sounds more fundamental than it is! In many cases, the TOC is used as an on-going monitor of change or lack of change in organic content.
What is measured when calculating Total Organic Carbon?
When completing TOC analysis, the following is measured:
• TC – Total Carbon
• TIC – Total Inorganic Carbon
• POC – Purgeable Organic Carbon
• NPOC – Non-Purgeable Organic Carbon
• DOC – Dissolved Organic Carbon
• NDOC – Non-Dissolved Organic Carbon
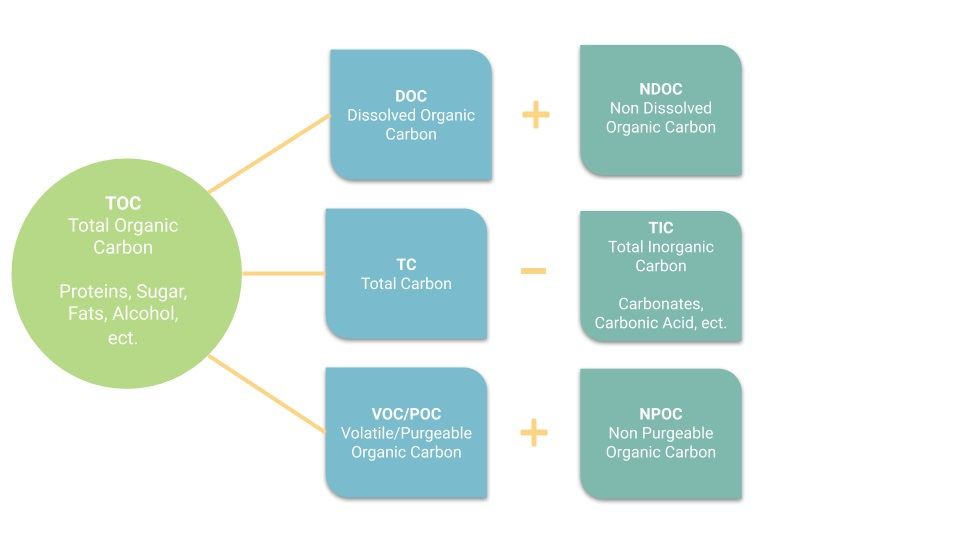
To calculate TOC, you can subtract the total amount of inorganic carbon from total carbon found. Alternatively, you can add Purgeable and Non-Purgeable Organic Carbon, or Dissolved and Non-Dissolved Organic Carbon. As sums, they look like:
TOC = TC - TIC
TOC = POC + NPOC
TOC = DOC + NDOC
How is Total Organic Carbon Measured?
TOC is measured at very different concentrations in a very wide range of systems. The table below gives an indication of Total Organic Carbon levels in various types of water. Levels vary widely within each type, but, broadly, they range from sub-ppb levels in ultra-pure water for laboratory and microelectronic applications up to hundreds of ppm in effluents and process streams.
For many of these systems, the TOC alone does not provide enough information. The carbon-containing compounds can be present in different forms and the proportions of each can be critical. A breakdown of some of these divisions is shown in the above diagram.
Dissolved organic carbon (DOC) is generally taken as that which will pass through a 0.45um filter. Large particle size TOC is classified as particulate or non-dissolved (NDOC). About 50 to 75% of DOC in natural waters is in the form of polymeric organic acids - fulvic and humic acids.
About 10% of the TOC is in colloids, mainly humic acids and various minerals. A further 10 to 20% are small molecules from the decomposition of organic matter.
PURELAB Ultrapure Water Purification Systems with built-in Total Organic Carbon monitoring are available from our Approved Partners – click here to find out more
Total Organic Carbon Analysis
TOC is universally measured by oxidizing the organic compounds present to forms which can be quantified.
A variety of oxidation and detection methods are used depending on the nature and concentration of TOC being measured and the analytical requirements (e.g. speed, sensitivity).
- High-temperature combustion at 1,200 °C in an oxygen-rich atmosphere. The CO2 produced is passed through scrubber tubes to remove interferences and measured by non-dispersive infrared absorption (NDIR).
- High-temperature catalytic oxidation at 680°C in an oxygen-rich environment inside tubes filled with a platinum catalyst followed by NDIR.
- Thermo-chemical oxidation with heat and a chemical oxidizer, usually a persulphate.
- Photo-chemical oxidation with UV and a chemical oxidizer, usually a persulphate.
- Photo-oxidation by ultra-violet (UV) light alone or with a catalyst. The UV oxidation method offers the most reliable, low maintenance method of determining TOC in ultra-pure waters.
The combustion methods (1 & 2) are mainly used for higher (ppm or greater) TOC concentrations or where there are high levels of particulates. Persulphate oxidation, enhanced by UV or heat, is widely used for laboratory TOC determination in many types of water from potable to pharmaceutical and electronic grades. The CO2 produced is usually measured by NDIR or by the change of conductivity that it produces when dissolved in a separate stream of pure water.
To exclude the effect of other oxidation products the gas may be passed through a membrane. Good oxidation can be achieved but a compensation method is needed to account for the blank from the reagent. TOC at ppb levels can be detected.
All TOC analyzers which measure CO2 will include CO2 from bicarbonates and carbonates unless this inorganic carbon (IC) is accounted for. IC can be removed by acidifying the sample to a pH value of two or less to release IC as CO2 which can be measured or vented to waste. The remaining non-purgeable TOC (NPOC) contained in the liquid is then oxidized releasing CO2 which is sent to the detector for measurement.
The situation is somewhat different for high purity waters with a low conductivity. Water with a sufficiently low conductivity (resistivity approaching 18.2 Mohm.cm) cannot contain significant concentrations of bicarbonates or carbonates (or other soluble salts) and no correction for IC is needed. As discussed before, all such trace-level measurements must be done on-line.
A number of TOC monitors have evolved to meet the need for rapid monitoring of low (ppb) TOC levels in high purity laboratory water systems. A rapid response is needed to ensure that the results are available and relevant to the relatively small volumes of water being dispensed. These monitors, generally, measure the conductivity of the water before and after oxidation; the change is calibrated against TOC content. Due to time limitations, oxidation is not always complete but is sufficient for monitoring purposes.
On-line TOC Analysis or in the Laboratory?
The great majority of samples for TOC are taken and analyzed in a laboratory. Where sub-divisions of TOC are needed, such as NDOC, samples are prepared before analysis.
On-line analyses are used for higher level TOC analyses when the required frequency or speed of analysis makes it preferable. On-line measurements are also essential for the measurement of TOC levels below 50ppb to avoid contamination. This contamination can be from extraneous TOC in the environment or containers but, more seriously, from carbon dioxide in the air which will rapidly dissolve in pure water. Carbon dioxide interferes with many of the techniques used to monitor trace TOC.
Water for TOC Analyzers and Monitors
Clearly, the purity of water needed for preparation of standards and blanks, for rinsing systems and cleaning components is very dependent on the concentrations being measured. For many, higher level, applications, carried out in the laboratory or on-line, type II water will be more than adequate.
Consistent quality will be more important than very high purity. For trace TOC determination (<50 ppb) which need to be carried out on-line, the water purity requirements are extremely stringent. As TOC will include carbon from all types of compound, they must all be virtually absent. Ultrahigh purity water with built-in TOC monitoring is strongly recommended.
What causes high total organic carbon?
High TOC can be caused by a number of factors:
- Environmental and Industrial Origins
- Organic materials from untreated wastewater, oil leaks, or industrial processes (e.g., dyes, solvents, and detergents) can introduce TOC into water systems.
- Poorly designed storage vessels or inappropriate materials can leach organics into the water.
- Residual contamination from previous samples in measurement systems can artificially increase TOC readings.
- Water Purification System Design
- Systems lacking essential components like reverse osmosis (RO), UV treatment, or final filtration may fail to adequately remove TOC.
- Overly complex designs may lead to maintenance challenges and inefficiencies without necessarily improving TOC control.
- Microbial Activity
- Microorganisms and bacteria can contribute to TOC through metabolic byproducts or breakdown of organic matter, especially in poorly maintained systems.
- Measurement Challenges
- Organic degradation intermediates (e.g., formic acid, formaldehyde) generated during advanced oxidation processes (AOP) like UV treatment can temporarily increase TOC levels.
- Human Error
- Errors in design, maintenance, or operation—such as using incompatible materials or neglecting cleaning and validation—can lead to elevated TOC levels.
What can measuring total organic carbon levels tell you?
Measuring TOC in ultrapure water systems provides critical insights:
- System Performance and Contamination Detection
- Evaluates effectiveness of purification steps (e.g., RO, UV, ion exchange).
- Alerts early contamination via elevated TOC levels from biofilm, degraded components, or feed water contamination.
- Regulatory Compliance
- Meets standards like ≤500 ppb for purified water and ≤10 ppb for ultrapure applications.
- Ensures compliance with pharmaceutical and semiconductor industry protocols.
- Source and Type of Organic Contamination
- Differentiates between TIC and NPOC/DOC; NPOC is a key indicator in ultrapure systems.
- Detects spikes from solvents, microbial byproducts, or system leachates.
- Process Control and Risk Mitigation
- Monitors byproducts like formic acid during oxidation processes.
- Helps minimize biofilm risks by maintaining TOC < 50 ppb.
- Analytical Method Insights
- NPOC methods (UV-persulfate + NDIR) are preferred in ultrapure settings due to minimal volatile organics.
What does it mean when TOC is high in water?
- High TOC indicates significant organic contamination from sources such as vegetation decay, microbial activity, or human activities.
- It signals potential water quality concerns that may require additional treatment.
- Can lead to the formation of harmful disinfection byproducts (DBPs) like trihalomethanes.
- Makes water treatment more challenging by increasing chemical demand and reducing treatment efficiency.
- In natural waters, can cause oxygen depletion and harm aquatic life.
- In industries like pharmaceuticals, may compromise product purity and efficacy.
- May cause water discoloration, which is undesirable for consumers.
How do you remove total organic carbon?
Several effective methods can be used to reduce TOC:
- Advanced Oxidation Processes (AOPs)
- UV/Ozone Treatment: Generates hydroxyl radicals to oxidize organics down to < 0.5 ppb.
- UV/Persulfate Oxidation: Effective across many applications but may struggle with high particulates.
- Ozone-based AOP: Reduces TOC and COD without producing sludge.
- Adsorption Methods
- Activated Carbon: Highly effective due to its large surface area for removing contaminants.
- Chemical Treatment
- Coagulation/Flocculation: Reduces TOC before sedimentation and filtration.
- Ion Exchange
- Removes dissolved ions and can complement other TOC reduction methods.
- Membrane Filtration
- Technologies like RO can significantly lower TOC levels.
- Thermal Methods
- High-Temperature Combustion: Oxidizes organic carbon at 1000–1200°C and converts it to CO2.
- Combination Approaches
- Physical-Chemical Treatment: Combines clarification, filtration, and adsorption for >95% TOC removal.
What contributes to total organic carbon?
TOC comes from both natural and synthetic sources:
- Natural Sources
- Decaying Organic Matter: From plants, animals, and microbial exudates.
- Natural Organic Matter (NOM): Includes humic/fulvic acids, amino acids, urea, VOCs.
- Atmospheric Deposition: VOCs from vegetation or biomass burning enter water through precipitation.
- Synthetic Sources
- Industrial Chemicals: Pesticides, fertilizers, detergents, etc.
- Hydrocarbon Contamination: From geological or industrial activity.
- Wastewater Effluents: Often introduce partially treated organics into waterways.
- Other Contributing Factors
- Soil Erosion: Carries organic matter from soil into water systems.
- Microbial Activity: Breaks down organic matter and releases DOC.
- Seasonal Variations: For example, autumn leaf fall increases TOC.
How does total organic carbon affect water quality?
High TOC in ultrapure water systems can:
- Compromise product quality and experimental accuracy.
- Support bacterial growth and biofilm formation.
- Increase conductivity and disrupt purity standards.
To mitigate these risks, regular TOC monitoring and use of advanced purification methods (e.g., UV oxidation at 185 nm) are essential.
