5 Chemical Feedstocks and their Sustainability
22 May 2025

The production of essential industrial chemicals is energy-intensive and environmentally impactful; the chemical industry currently accounts for ~5 % of global greenhouse gas emissions [1]. Addressing sustainability concerns becomes increasingly crucial as global demand rises, driven by expanding industrial sectors and human population. Exploring alternative production routes from renewable sources, optimising processes, integrating renewable energy, and adopting circular economy principles can minimise greenhouse gas emissions and resource depletion. These sustainable practices are vital to mitigate the environmental consequences of industrial demands. Sulfuric acid, sodium hydroxide, nitrogen, ethylene and propylene are 5 of the most common chemicals used industrially [2], so these are a good place to start to look at producing and using chemicals sustainably.
Sulphuric Acid
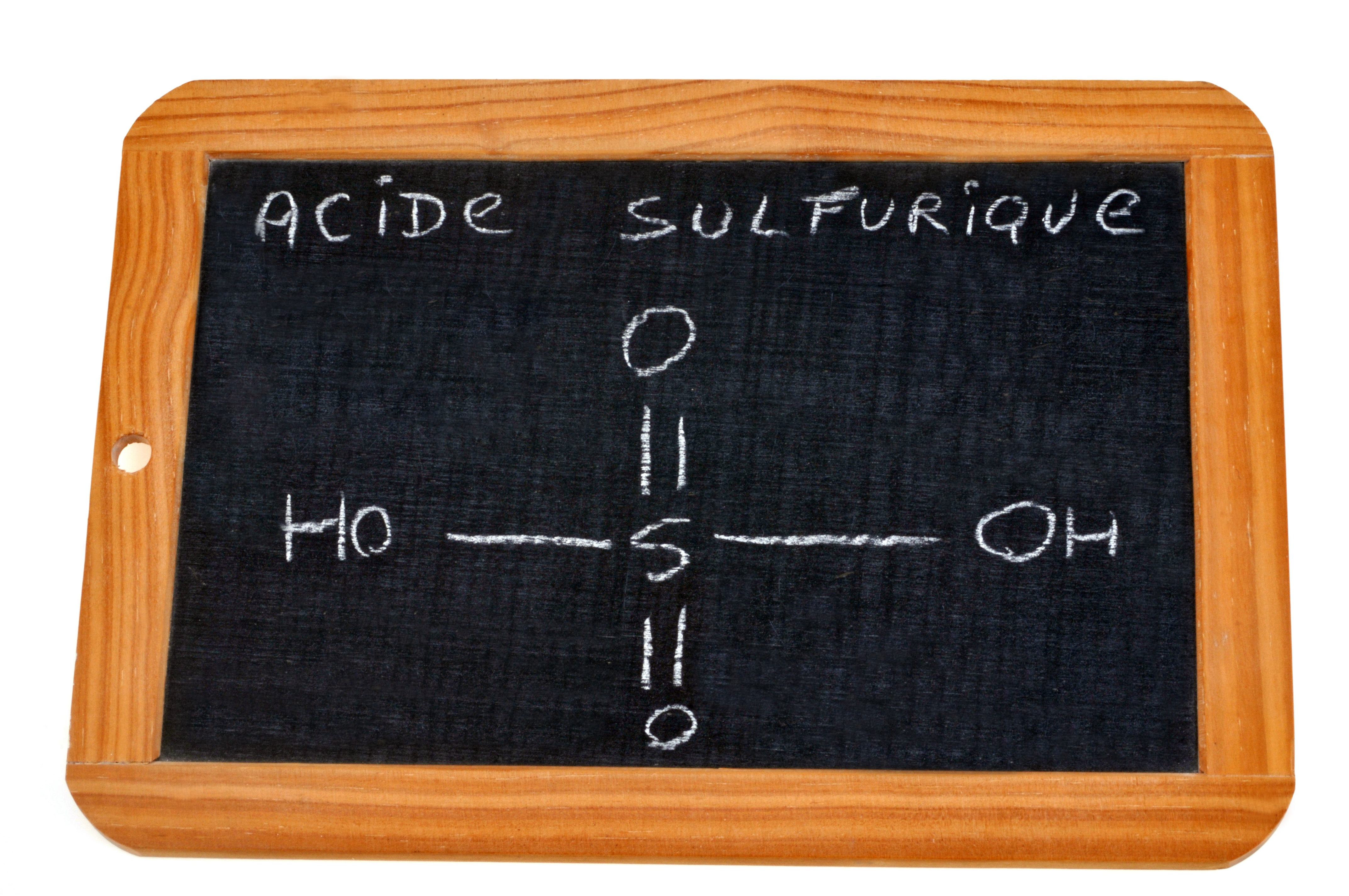
Sulphuric acid (H2SO4) is a vital industrial chemical with a wide range of applications, including the production of fertilisers, chemicals, and pharmaceuticals, as well as in various industrial activities such as metal processing. Its importance stems from its strong oxidising and dehydrating properties, making it an essential component in numerous chemical reactions. Further, with over 80% of the current global sulphur supply being sourced from the desulphurisation of fossil fuels, the foreseen ~60% increase in demand of sulphuric acid with “rapid growth in the green economy and intensive agriculture” by 2040 could prove to be very problematic [3].
Traditionally, sulfuric acid has been produced through the contact process, which involves the oxidation of sulphur dioxide (SO2) to sulphur trioxide (SO3), followed by the absorption of SO3 in water [4]. This process has significant environmental impacts, such as the emission of 88.92 kg CO2 equivalent(eq)/tonne of H2SO4 produced, emissions of sulphur oxides which cause acidification, and in a life cycle assessment, a total of 11,300 MJ-eq is required to produce 1 tonne of H2SO4 [4]. To address these sustainability concerns, researchers are exploring alternative avenues, such as the regeneration of spent acid [5], which also reduces the detrimental effects of its disposal on the environment and structures including metallic corrosion, or disturbance of soil or surface water pH [6]. Researchers are also speculating the development of methods to recover sulphur from sulphate salts using sulphur-oxidising bacteria [3]. While such sulphur recovery methods remain largely hypothetical at the current stage, this type of innovative approach is necessary to drive critical changes and improvements in industrial processes.
Sodium Hydroxide

Sodium hydroxide (NaOH), commonly known as caustic soda, is a versatile and essential chemical compound with numerous industrial applications. It is widely used in the production of soaps, detergents, paper, textiles, and various other chemical processes [7]. Sodium hydroxide also plays a crucial role in the treatment of industrial wastewater and the production of aluminium and biodiesel.
Traditionally, sodium hydroxide has been produced through the chlor-alkali process, which involves the electrolysis of brine (sodium chloride solution) [8]. This can have significant environmental impacts, such as the generation of chlorine gas, historically the use of mercury (although this is being phased out), and similar to the production of sulphuric acid, is energy-intensive: 7,200 - 9,000 MJ - eq is required to produce 1 tonne of NaOH via the chlor-alkali process [9]. Further, efforts are being made to optimise the chlor-alkali process by implementing new technologies, such as oxygen-depolarized cathodes and ion-exchange membrane electrolyzers, which can significantly reduce energy consumption and minimise the formation of byproducts [10]. Furthermore, the integration of renewable energy sources, such as solar and wind power, into the electrolysis process can further enhance the sustainability of sodium hydroxide production [10].
Nitrogen

Nitrogen gas (N2) is an essential industrial gas with numerous applications across various sectors, including food processing, pharmaceuticals, electronics, and chemical manufacturing [10]. Its inert nature and ability to displace oxygen make it invaluable for creating controlled atmospheres, preserving perishable goods, and ensuring safe handling of reactive materials.
Traditionally, nitrogen gas has been produced through the fractional distillation of liquefied air, a process that separates the components of air based on their boiling points [11]. Alternative production methods are being explored to minimise energy consumption and hence reduce greenhouse gas emissions, these include membrane separation and pressure swing adsorption (PSA). These techniques offer improved energy efficiency (“PSA uses about 28% less energy than liquid nitrogen created by traditional air separation” [12]) and hence reduced environmental impact.
Investigation to optimise the nitrogen cycle in natural ecosystems is also occurring, as nitrogen plays a crucial role in various biogeochemical processes. Reactive forms of nitrogen, compounds where it is bonded to oxygen, hydrogen or carbon, are essential for life, and the balance between nitrification, denitrification and nitrogen fixation has been broken, particularly through industrialisation, leading to excess nitrogen pollution, and strains on resources. The importance of dealing with this issue has led to efforts at the highest level with world leaders at the United Nations Environmental Assembly discussing sustainable nitrogen management in 2019, and later agreeing to ““halve nitrogen waste” from all sources by 2030 in the Colombo Declaration on Sustainable Nitrogen Management” [13].

Ethylene and Propylene
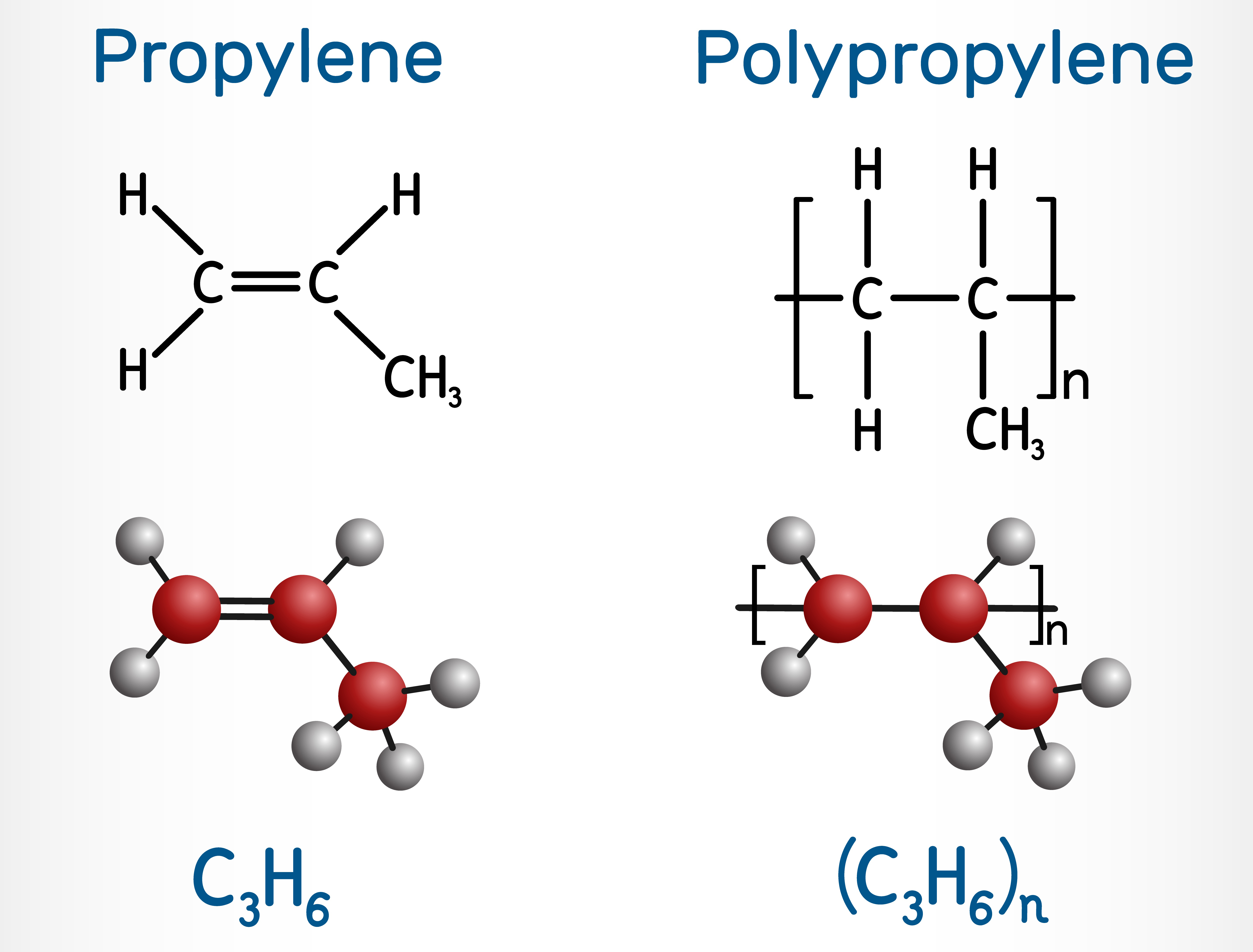
Ethylene (C2H4) is a crucial chemical feedstock with immense industrial significance. Its versatility stems from its ability to undergo various chemical transformations, leading to the production of a wide range of valuable products, including polyethylene, ethylene oxide, ethylene dichloride, and vinyl acetate [14].
Similarly, propylene (C3H6) serves as a building block for a wide range of products, including polypropylene, propylene oxide, and acrylonitrile [15]. Polypropylene, in particular, is a versatile thermoplastic polymer widely used in packaging, automotive components, and consumer goods due to its excellent mechanical properties and chemical resistance.
Traditionally, ethylene has been produced through steam cracking of fossil-based feedstocks, such as naphtha and ethane [16] with propylene produced as a byproduct [17]. As above, this process is hugely energy-intensive (26 GJ/tonne ethylene [18]) and contributes significantly to greenhouse gas emissions, raising concerns about its environmental impact. To address these environmental concerns, researchers are exploring alternative routes for ethylene and propylene production from renewable sources, such as bioethanol and biomass [19, 20]. One promising approach involves the catalytic dehydration of bioethanol, which can be obtained from fermentation of biomass feedstocks like lignocellulosic materials and agricultural residues [21]. Additionally, the development of novel catalysts and process intensification strategies aims to improve the efficiency and sustainability of both ethylene and propylene production [14, 15, 20]. Furthermore, the recycling and reuse of polypropylene and other propylene-based products can contribute to a more circular economy, reducing the demand for virgin materials and minimising waste [22, 23].
Conclusion
Sustainability has become imperative in all sectors, including the chemical industry, as we grapple with the environmental consequences of human activities. Chemists are ideally positioned to develop the impactful changes that are required. While some of the approaches detailed in this blog are still in the early stages, innovative thinking and a commitment to driving critical changes in industrial processes are necessary to meet the world's industrial needs while minimising environmental consequences. By embracing sustainable practices, the chemical industry can play a vital role in achieving a balance between economic growth and environmental protection.
ELGA Labwater, a Veolia company, is at the forefront of sustainable water purification solutions. Aligned with Veolia's core purpose of saving and regenerating water resources, ELGA brings deep expertise in high-purity water and waste treatment to laboratories worldwide. Their products feature reclaimed materials, high-efficiency water purification, and energy-efficient designs. ELGA's commitment to sustainability extends to renewable power usage and low-emission manufacturing processes. Together, ELGA and Veolia are driving innovation in water purification technology, contributing to a more sustainable future for scientific research and industrial applications.
Reference
- Gabrielli et al., 2023, ‘Net-zero emissions chemical industry in a world of limited resources’, One Earth, 6(6), 682-704, DOI:10.1016/j.oneear.2023.05.006
- https://www.chemicals.co.uk/blog/5-common-industrial-chemicals#summary
- Maslin et al., 2022, ‘Sulfur: A potential resource crisis that could stifle green technology and threaten food security as the world decarbonises’, The Geographical Journal, 188(4), 498-505, DOI:10.1111/geoj.12475
- Marwa et al., 2017, ‘An environmental life cycle assessment of an industrial system: Case of industrial sulfuric acid’, International Journal of Energy, Environment, and Economics, 25(4), 255-268
- https://www.up.com/customers/track-record/tr081622-circular-economy-sulfuric-acid-ecoservices.htm
- Agarwal et al., 2023, ‘Remediation and recycling of inorganic acids and their green alternatives for sustainable industrial chemical processes’, Environmental Science: Advances, 2, 1306-1339, DOI:10.1039.d3va00112a
- https://www1.eere.energy.gov/manufacturing/resources/chemicals/pdfs/profile_chap6.pdf
- https://www.veoliawatertech.com/en/solutions/technologies/whittier-filtration/chlor-alkali
- Kumar et al., 2021, ‘Caustic soda production, energy efficiency, and electrolyzers’, ACS Energy Letters, 6(10), 3563-3566, DOI:10.1021/acsenergylett.1c01827
- Du et al., 2018, ‘Sodium hydroxide production from seawater desalination brine: process design and energy efficiency’, Environmental Science & Technology, 52(10), DOI:10.1021/acs.est.8b01195
- https://nigen.com/how-is-nitrogen-gas-produced-for-industrial-applications/#:~:text=Fractional%20Distillation,-Fractional%20distillation%20is&text=This%20nitrogen%20gas%20plant%20process,hundred%20percent%20purity%20(99.999%25).
- Froehlich, 2013, ‘A sustainable approach to the supply of nitrogen’ https://www.parker.com/literature/Balston%20Filter/IND/IND%20Technical%20Articles/PDFs/Sustainable%20Approach%20to%20N2%20Supply.pdf
- https://www.unep.org/nitrogens-turn
- Chauhan et al., 2023, ‘Advancements in environmentally sustainable technologies for ethylene production’, Energy & Fuels, 37(17), DOI:10.1021/acs.energyfuels.3c01777
- Phung et al., 2021, ‘(Bio)propylene production processes: a critical review’, Journal of Environmental Chemical Engineering, 9(4), DOI:10.1016/j.jece.2021.105673
- Kim et al., 2023, ‘Sustainable ethylene production: recovery from plastic waste via thermochemical processes’, Science of The Total Environment, 903, DOI:10.1016/j.scitotenv.2023.166789
- Tahkamo et al., 2022, ‘Life cycle assessment of renewable liquid hydrocarbons, propylene, and polypropylene derived from bio-based waste and residues: evaluation of climate change impacts and abiotic resource depletion potential’, Journal of Cleaner Production, 379, DOI:10.1016/j.jclepro.2022.134645
- Worrell et al., 2000, ‘Energy use and energy intensity of the U.S. chemical industry’, United States: N. p., DOI:10.2172/773773
- Kim et al., 2021, ‘What is the best green propylene production pathway?: technical, economic, and environmental assessment’, Green Chemistry, 19, DOI:10.1039/D1GC01791H
- Nyhus et al., 2024, ‘Green ethylene production in the UK by 2035: a techno-economic assessment’, Energy & Environmental Science, 17, 1931-1949, DOI:10.1039/d3ee03064d
- Chen et al., 2023, ‘Ethylene production: process design, techno-economic and life-cycle assessments’, Green Chemistry, 26, 2903-2911, DOI:10.1039/d3gc03858k
- https://www.veolia.co.uk/services/recycled-uk-recycling-materials
- https://www.palmetto-industries.com/recycling-polypropylene/
How can we help?
Interested in sustainable solutions for your business? Contact our team to explore tailored strategies
Contact Us
Related Content
#e95f47
